お知らせ・お役立ち便覧 NEWS
有機溶剤
2024.11.12
メタノールについて
目次
メタノールとは
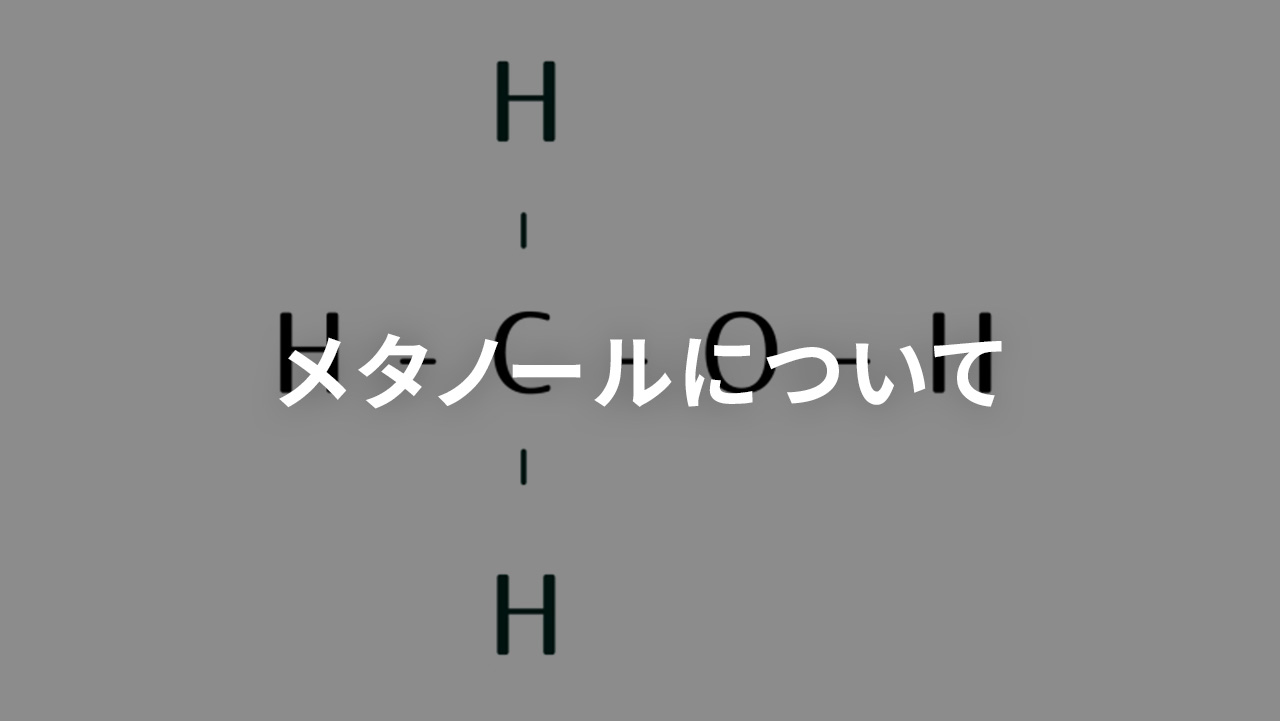
メタノールはアルコール系溶剤の一つで、別名、メチルアルコール、メチール、木精、とも呼ばれます。
化学式はCH3OHです。
理科室などでよく見かける「ホルマリン漬け」のホルマリンの原料になったり、アルコールランプなどに使われることが多いです。
また、最近は燃料電池の水素供給源としても注目を集めています。
メタノールとエタノールの違い
★合わせて読みたいオススメ記事★
メタノールとエタノールの違いの一つは、製造方法にあります。
メタノールはその別名の通り、木材から精製していた為「木精」と呼ばれています。
最近では天然ガスや石炭ガスを精製することによって作っています。
一方で、エタノールはデンプンや糖を発酵させて作ったり、エチレンを原料に触媒を使った水和反応で作っています。
また、大きな違いとして、メタノールは人体に有害です。
一部のエタノールはお酒の中にも含まれており飲まれることもあると、エタノールの紹介記事でも書きましたが、メタノールは全て、絶対に飲んではいけません。
特に視神経にダメージを与えることが有名で、一部では「メチル(目散る)アルコール」とも言われるほどです。
摂取してしまうと失明の危険性だけでなく、最悪死に至る可能性もありますので、十分に注意して下さい。

メタノールの危険性
絶対に飲んではいけない
前述の通り、メタノールは絶対に摂取してはいけません。
エタノールの場合、摂取すると体の中の「酵素」によって酸化され、「アセトアルデヒド」という物質になります。
メタノールの場合は、酵素による酸化で「ホルムアルデヒド」という物質になります。
アルデヒドはどちらも有害なのですが、そこから更に酵素によってそれぞれ、
「アセトアルデヒド」は「酢酸」に
「ホルムアルデヒド」は「蟻酸」に分解されます。
酢酸はご存知の方もいると思いますが、「お酢」の酸味の主成分となるものです。
(ただ、このとき全てが分解しきれないとアセトアルデヒドが残ってしまい、それが二日酔いの頭痛や吐き気の原因になります)
蟻酸は酢酸とは対照的に有害で、これこそがメタノールを飲んではいけない原因なのです。
その漢字の通り、昆虫のアリの一部の種類が持っている毒で(※全てのアリが持っているわけではない)
この毒を外敵にかけて獲物を捕まえたり、身を守ったりします。
皮膚を損傷したり、体内に毒が入ると視神経を侵してしまうなどの症状がでます。
そのため、メタノールを摂取してしまうと体内で蟻酸が生じ、その毒で問題を起こしてしまうのです。

引火性が高い
メタノールはアルコール類の中でも最も引火点・沸点が低い溶剤です。
沸点が低く、揮発しやすい上、常温でもすぐに火がついてしまいます。
また、メタノールに火が付いた際の炎は、エタノール同様青白く見えづらいため、発火していてもすぐに気付けず発見・消火が遅れ大参事になることも考えられます。
火気にはくれぐれも気を付けるとともに、メタノールの蒸気が充満しないよう、しっかり換気をしてください。
もし発火してしまったら
水で消火、または窒息消火します。どちらでも大丈夫です。
炎が見えづらい為、特に明るい場所では火がまだついているのか鎮火したのか分かりづらいため、陽炎のような空気の揺らめきなどにも注意して念入りに消火することが大事です。
保管について
メタノールは劇物であり、引火の危険もあるため、密閉した容器に入れ、鍵の掛かる場所で施錠して保管します。
万が一にも、容器が密閉しきっておらず中身が揮発し保管所内にメタノールが充満することのないように換気も徹底してください。
関連製品
メタノールに関する質問
※2024年11月12日更新
以前、ページのコメントに寄せられた疑問・質問とその回答をご紹介します。
(※一部編集・抜粋しております)
質問No.01~10
※「▼」をクリックすると質問が表示されます。
1、水に良くとけますか? 放置した場合は分離しますか?(比重が水より軽いため)
2、水処理実験で生活排水のBOD濃度が低いので補てん(生活排水:メタノール=1:1)として使用したいのですが注意点ありますか?
1.メタノールは水に溶けやすいので放置しても分離しません。
2.メタノールと水を混ぜるとBODは上がりますが、メタノールには毒性がございますので、毒性に注意してください。
先日、海外のメタノール死亡事故のニュース見ました。
調べてみると、ウォーシャー液など結構身近なものに使われてますよね。こういうものも誤飲した場合、死亡するなど危険があるのでしょうか?
メタノールの誤飲による死亡の確率は量の大小もあります。
誤飲した場合は即座に病院へ行き医師の診察を受けてください。
勿論、メタノールに限らず他の溶剤でも同じように、誤飲してしまった場合はすぐ病院に行って医師の指示に従ってください。
メタノールにもエタノールやIPAのような除菌、殺菌効果がありますか。
具体的にはウエスにしみ込ませモノの汚れを洗浄、除菌したりしたいのですが、どうでしょうか。
今まで、職場で手に入ることもあり、一番毒性が強いならそう言った効果もあるであろうと思い使用していました。
メタノールの毒性というのは人に対しての毒性であり、細菌やウイルスに対してはまた違う毒性になります。
そのため毒性が高いと言われているから細菌やウイルスにも効果があるというわけではありません。
なるべく人に害が少なく、細菌やウイルスに効くものをご使用いただきたいです。
除菌効果の有無について回答していただけないでしょうか?
物性的にエタノールに近い(脂質を溶かす)のであれば、エタノール同様に細胞壁やコロナウィルスのエンベロープを破壊すると思われますが。
肌ではなく物を消毒する目的においては、漂白剤やオキシドール、ベンジン等で代用するよりかは安全のような気がします。
「なるべく人に害が少ないもの」を使いたいのは、言われなくても誰もがそう思っています。入手できないときにどうしたらよいのか、そのヒントを得るために質問しているので、その点を汲み取った回答をいただけるとありがたいです。
メタノールは脂質を溶かす性質を持つためエンベロープを破壊しますが、細胞壁は壊すことはできません。
物品の消毒に関しては、メタノールはオキシドールやベンジンよりも毒性が強く、さらに誤飲の可能性もありますのでそのような用途には絶対に使用しないでください。
エタノールが入手できない場合IPAや次亜塩素酸ナトリウムなど抗ウイルス作用の強い消毒薬で洗浄ください。消毒に関してご不安があられましたら、厚生労働省様より文書が掲載されておりますので、そちらも併せてご確認いただければと思います。
https://www.mhlw.go.jp/content/000548441.pdf
メタノールの人体への摂取ばかり気にされてますが、メタノールはオキシドール以上の消毒・殺菌効果があるということでしょうか。
気休めでも(換気しながら)部屋の手に触れる場所や持ち物の清拭をしたいのですが、危険でしょうか。
メタノールは人体への有害性が高いですが、それがウイルスへの有害性が高いことにはなりません。
例えば玉ねぎは、猫が食べたら毒ですが、人間が食べても害がないのと同じで、対象それぞれに毒になるもの、ならないものが異なります。
絶対に使用しないでください。
消毒用アルコール(エタノール)が入手できないでいます。
燃料用のメタノールが混ざったアルコールからエタノールだけ分離するのは難しいですか?
もともと飲めなくする目的でメタノールが混ぜられているものです。ご家庭で簡単にエタノールだけ抽出できてしまうとその役目を果たせなくなってしまうのです。
そのためご家庭でエタノールの抽出、というのは非常に困難かと思います。
エタノールが高いのは酒税がかかってるからでしょうか?メタノールだから安いわけじゃない、飲めないから酒税がかからないかですか?
おっしゃるとおり、エタノールも、添加物等で飲めなく加工し、酒税がかからないようにした「変性エタノール」もございますので、こちらも酒税がかからないので安く販売されております。
消毒用エタノール、無水エタノールが手に入らないため、燃料用のバイオエタノールで代用できないかと考えています。
精製水で希釈して、手指消毒用に使用できないかと考えているのですが、この程度のメタノール含有量でもメタノールは有害なのでしょうか?それとも、飲用しなければさほど害のないものなのでしょうか?
メーカーに成分を問い合わせたところ、90%程度のバイオエタノールと、10%程度のメタノール、とのことでした。
10%でも危険性はございます。
また、飲用しないから大丈夫と思っていても、(手指に塗布後乾燥しきっていない状態で顔まわりを触ってしまい摂取してしまうなども含め)誤飲の可能性が発生致しますのでどうかご遠慮いただけますようお願い申し上げます。
亡くなった父の所有していた物の中にメタノールがありまして、劇物と書いてあったので早めに処分をしたいのですが、どうしたらいいのかわからず困っています。
メタノールはどのような手段で処分したらいいのでしょうか?
毒劇物の処分は、各自治体によって異なるため、「こうです」とご案内することができません。
そのため、お住まいの地域の最寄りの自治体様へお問い合わせ頂きたくお願いいたします。
エタノール86w/w%含有、メタノール2.8w/w%含有、n-プロピルアルコール、8.8w/w%含有、イソプロピルアルコール 1.1w/w%含有をコロナの為に身の回りの消毒へ使うのは難しいでしょうか?
台やパソコンや携帯などの消毒にと思っていました。
メタノールはアルコール系溶剤の中でもトップクラスに人体への害が高いものです。
(半がわきの状態のものを誤って口に含んでしまうことも含め)誤飲の可能性が非常に高く、そのような事例も過去に沢山起きているため、
絶対におやめくださいませ。
質問No.11~20
※「▼」をクリックすると質問が表示されます。
全国的に無水エタノールが不足しており、水に非常に弱い電子機器の洗浄に難儀しているのですが、メタノールや白ガスで代用できるものでしょうか?
汚れの種類にもよりますが、白ガス(ホワイトガソリン)で代用可能かと思います。
メタノールは誤飲の可能性がございますので、お勧め致しません。
通販でエタノールを買えたのですが、最近は詐称品が出回ってもおかしくないくらい品薄なのに購入できたため、本当に中身がエタノールなのか疑心暗鬼になっております。
もしもメタノールのような毒性のあるものだったと考えると不安で仕方ないです。
エタノールとメタノールの確認方法がご教示いただけませんでしょうか。
エタノールにメタノールが混ざっている場合、一般のご家庭で簡単に確認する方法はございません。
ただ、エタノール製剤の原料となりますエタノールにつきましては在庫がひっ迫しているというお話を聞きませんので、詐称品が出回る可能性は低いかとみております。
ご心配であれば、ご購入前に標記のメーカー名をお調べになり、メーカー様にお問い合わせなさったり、メーカー様の実績をお調べになるのが確実かと思います。
メタノールを身近で行える使い方があれば教えてほしいです。
メタノールの身近な使い道でございますが、メタノールは毒物劇物取締法の劇物に当たりますので一般的な使用はご遠慮いただきたくお願いいたします。
先日ドラッグストアで購入したアルコール除菌剤について質問です。
用途は「手が触れる箇所の除菌」で、テーブル、イス、手すり、トイレなどに使用できると書いてあります。
成分は「エタノール80%以上、メタノール、ノルマルプロピルアルコール」です。この記事を読ませていただきましたが…やはり使用しない方がいいのでしょうか。
弊社としてあまり他社様の製品について言及するのは控えさせていただきたいのですが、もしわたくしならば使用を控えたいと思ってしまいます。
もしご使用される際の注意点
●火気厳禁(アルコール濃度が非常に高いように思いますので、くれぐれも火気や静電気にご注意ください。)
●使用時は換気をする
●誤飲に注意する
塗布後はしっかり乾燥させてください。
塗布した場所のメタノールが【乾いた】場合にも同じ様な人体への影響はあるものでしょうか?
例えば、①触った手で目をこするとか、②乾いたものを熱してその時の見えないガスを吸引いてしまうとか。
塗布後、完全に乾いてしまえば、メタノールの成分が塗布面に残ることはありません。
そのため、メタノールが過去に塗布された乾燥した面を手にはメタノール成分はつきません。
ですが乾燥時に空気中にメタノール成分が揮発していることになりますので、換気をしっかりして、保護具を正しく着用して作業ください。
コロナウィルス予防のためマスクをしていますが、気休めですがマスク使用後にメタノールをかけて数時間たって完全に乾いてから再利用しています。
そこでご質問ですが、メタノールが完全に乾燥した後でも毒性があるのでしょうか?
他の方のご質問からコロナに効果があるのかは不明ということは承知しております。
完全に乾けば問題ありませんが、メタノールを使用する際は必ず保護具を着用し換気の良いところで作業を行ってください。
換気の悪いところで保護具を着用せずに作業を行われますと、揮発しているメタノールを吸い込み大変危険です。また、メタノールは妊娠や出産・授乳機能に影響のある物質の一つとされており、
メタノールを使用する屋内労働環境では女性は就労禁止になっていますので、そちらも念頭に置いて作業いただければと思います。
アルコールストーブを作ったのですが、燃料としてメチルアルコールを使う予定です。
ここで質問なのですが、メチルアルコールに火をつけ気化?って言うのかわかりませんが、蒸発したメチルアルコールを吸ってしまった場合、体に害はあるのでしょうか?
もう一つ、消毒用エタノールを燃料として使うことに危険性はありませんか?
メタノールが完全に燃焼してしまえば問題ないのですが、気化したものや不完全燃焼を起こしたものを吸い込むと害があります(不完全燃焼の場合一酸化炭素が有害)。
消毒用エタノールは濃度100%であれば問題ありませんが中には希釈されているものがございますので、そちらですと燃えづらいかと思います。また、メタノール100%の場合、炎が見えづらくなります。
通常の燃料用アルコールにはIPAを混ぜていて、これによって炎が見えやすくなっています。
メタノールに触れた時について、いくつか質問があります。
1、メタノールは危険なのは承知してるのですが、よく料亭などの鍋料理の固形燃料や、BBQなどの着火剤もメタノールですよね?
あれも間違えて食べてしまったり、お箸でつついたりしたあとに舐めたりしたら、メタノールを誤飲した時と同じようになるんですか?
2、シックハウスの原因となるホルムアルデヒドを体内に取り込んだ時と、メタノールを取り込んだ時の症状は同じなんですか?
物質が元々同じような気がするんですが、ホルムアルデヒドは視力の症状は見かけないのは何故なんでしょう?
またよくホルムアルデヒド除去スプレーが売っていますが、その効果は本物ですか?となるとメタノールが揮発してる所でも有効なんでしょうか?
1も2も濃度が薄いので、体に大きな変化はありません。
除去スプレーに関しては、そのような製品を、お恥ずかしながら弊社で見たことも聞いたこともなく、どのようなものなのかわかりませんで、よければメーカー様へご確認ください。
先日、車のウォッシャー液が不注意で車内にかかってしまったのですが、揮発したら問題ないですか?付いたかもしれない服は洗濯でいいでしょうか?
メタノールは揮発し、空気が循環してしまえば問題ありませんが、空気中に揮発した濃度の濃いメタノールを吸うと危険ですのでお気を付けください。
また、ウォッシャー液に含まれる別の成分で不揮発成分(界面活性剤など)がありますので、洗濯をお勧め致します。
新型コロナが大流行しているため、海外製造のエチルアルコール(95%)を買いました。
しかしこの記事を読んで、ひょっとしたらメチルアルコールではないかと不安になりました。あまりに安かったからです。
メタノールとエタノールを見分ける方法などありませんか?
例えばエタノールにメタノールが混ざってしまっている場合、簡単に見分ける方法はありません。
もしメタノール単体であれば、においが違ったり、火をつけた際の色で違いがわかりますが、比べるものがないとなかなか難しいです。
メーカー様へメチルアルコールが含まれていないか確認なさってください。
質問No.21~30
※「▼」をクリックすると質問が表示されます。
衣料やソファーなどにも利用する芳香消臭スプレーにもメタノールを数パーセント含んだ変性アルコールが20%程度含まれていますが、こちらは問題ないのですか?
現在メタノール変性のアルコールは少なくなっており、どのような製品なのかは弊社ではわかりかねますが、メーカー様へご確認をお願いいたします。
ノルマルプロピルACLとメタノール1.1%について質問です。下記の成分の場合、どのような特性や危険性があるのでしょうか。
除菌アルコールを探していたら、下記の成分のエタノール溶剤を2つ、みつけました。
その1)
メタノールを含まない、除菌掃除にとの記載でしたので、20%の水とグリセリンを混ぜて、手指の除菌に使おうと考えました。しかし、ノルマルプロピルアルコールが含まれているようです。
これは、どんなアルコールなのでしょうか。
——-
エタノール 80-90%
IPA イソプロピルアルコール 1-5%
NPA ノルマルプロピルアルコール 5-10%メタノールは含まれておりません。
脱脂や洗浄、お掃除にご使用頂けます。工業用の脱脂剤として製造されたものです。
———
その2)
これも20%の水を加えてドアノブなど消毒しようと考えていますが、メタノール1.1%をさらに薄めると危険度はどのくらいなのでしょうか。メタノール原液ではないので直接、目や口に入れなければ大丈夫と思いました。
————
エタノール80-90%
メタノール1.1%
イソプロピルアルコール15-19%
———-
もちろん、2つとも工業用ということは承知の上です。
NPA(ノルマルプロピルアルコール)は炭素数が3のアルコール類で、イソプロピルアルコールに比べて水酸基が点く位置が違います。
>>NPA(ノルマルプロピルアルコール)について
上記リンクより安全データシート(SDS)をご確認いただけます。メタノール含有のアルコールについてですが、吸い込んでしまったり、飛沫が付着してしまったりする恐れがありますのでご遠慮ください。
また、薄めた本人はメタノールが入っている危ないものだとわかっていても、他の方がわからないで誤った使用方法をしてしまったり、誤飲してしまった恐れがあります。
弊社ではお勧めできません。
先日、手指の消毒用にとのことで貰ったものが、実はメチルアルコールでした。
それがわかるまで2、3週間程、アトマイザーに入れて使用しており、手指にかける時に少し吸い込んだりしていたと思います。体調等の異変はないのですが、子供も使用していたため心配しています。
使っていて、とても揮発性が強かったため、途中から8:2で水で薄めて使用していました。今の所、体調に異変はないのですが病院等に相談したほうが良いのか悩んでいます。
子供たちの手は特に荒れてはいませんが、私の手が荒れておりガサガサしています。
お医者様へ掛かって、メチルアルコールを使用した旨と吸い込んでしまったおそれがある旨をお伝えください。
一時的にでも体内に取り込まれる量が多いと、体に影響がでる恐れがあります。
メチルアルコールを使用する作業場では、法律上女性が就労禁止になるほど生殖毒性も強いものなので、一度検査なさってください。
メタノール14キログラムx5缶を2ヶ月間隔で使用しています。毒性の強さからこれまで代用できる溶剤を探してきましたが見つかりません。
使用に際しては強力換気をしていますが代用できる溶剤はあるのでしょうか。
具体的にはアクリレート系紫外線硬化樹脂の洗浄除去のためメタノール14キログラムx5缶を2ヶ月間隔で使用しています。
圧力噴霧除去で使用しています。30年間この方法での使用ですが、今日まで身体にとくべつの変化は起きていません。
3Dプリンターであれば、硬化前の樹脂であれば弊社製品で落とすことができるかもしれません。
詳しくは弊社までお問い合わせくださいませ。
メタノールが他の物質と混ざっている場合でも、メタノールの揮発性は変わらないのですか?
例えば、家庭用エアゾル製品に含まれていたとして、その製品が残っていたらその中のメタノール成分はそのままなのですか?揮発してしまうのでしょうか?
不揮発成分と混ぜられると同じく揮発性しにくくなるのでしょうか?
多少遅くなることはありますが、揮発します。
ただ、塗布後の液を見てその中に「まだメタノールの成分が残っている!」「すべて揮発してしまってもう残りの液しか残っていない!」ということはわからないので、
液が残っていたら、メタノールが含まれていると思ってください。
また、揮発中はその周辺の気体に含まれるメタノール濃度が上がりますので、しっかり換気をして、期待を吸い込まないようマスクや保護具を装着してください。
ALCHOL 90%と書かれた商品で手指消毒やテーブルやドアノブの消毒などにかなりの頻度(既に2L)で1か月使用しています。
メーカーには原料問い合わせてみますが、これだけの使用の場合、既に何らかの症状が出るものでしょうか?
後進国在住でALCHOL 90%と書かれたものを薬局で購入しました。
特に体調等、手のひらも変わった様子はありません。メタ―ノール使用の場合は手荒れが発生しない場合もあるのでしょうか?
これだけの使用の場合、既に何らかの症状が出るものでしょうか?急性の場合は比較的症状が早いようですが、慢性の場合は症状でるのが遅いのでしょうか?
手荒れだけが害なわけではなく、
例えばメタノールには視覚器への毒性や、生殖毒性もありますから、目に見えないところで体に害が及ぶ可能性もあります。
また、例えばエタノールでかぶれる方もおられるように皮膚などの耐性には個人差があります。
コロナ対策のため、アルコール類を購入しましたが、メチルアルコールが37パーセント含まれています。59パーセントはエチルアルコールですが、薄めて使うことは可能でしょうか?
もし危険な場合の処分方法を教えていただければ、助かります。
ご使用にならないでください。おそらくそちらは燃料用としてお使いいただくものです。
万が一誤飲をしてしまった場合、最悪死に至ります。
誤飲をしないと思っていても、例えばその液にメタノールが含まれていると知らない方が誤飲をするケースや、あるいは揮発しきる前の液が口に入る、あるいはメタノール濃度の高い空気を吸い込んでしまうと危険です。
処分方法は、お住まいの地域の自治体様ごとに変わりますので、自治体様へお問い合わせください。
エタノール(エチルアルコール99.5)を使用して、機械の洗浄をする作業を数日行いました。顔近くで作業していた為、揮発したエタノールをけっこう吸引したと思います。
数日前から、動悸、吐き気を感じることがあるのですがエタノールが原因ということはありますか?
ただの体調不良かと思っていたのですが、本日エタノール作業中に吐き気と咳があったので、少し気になりました。
可能性としては十分ございます。
特にお酒に弱い方であれば、症状は出やすいかと思います。
揮発したエタノールを吸い込むことは飲酒と同じような作用をもたらしますので、作業時は防毒マスクを着用し、換気をしっかりし、揮発成分を吸い込まないよう作業くださいませ。
揮発した場合について、健康面への危険性について質問です。
先日コーヒーサイフォン用のアルコールランプに燃料用エタノールを入れ、食器棚に置いておいたのですが、気づいたら使わない間に揮発してなくなっていました。
容量は20mlくらいで、燃焼部分に被せる金属の蓋もしておきました。
食器棚の開け閉めはほとんどしないのですが、ガラス戸に少しだけ隙間があり、すぐ近くにいつも座っている状態です。
ガラス戸の隙間から揮発した物を吸い込んでいた状態なのかと思い、不安になりました。
健康面の心配をした方がいいでしょうか?
被せるだけの蓋であれば密閉できていないことが多いので、密閉できていない場合揮発します。
今回の場合20ml程度でございますので、お体に問題なければ大丈夫かと思いますが、耐性に関しては個人差がありますので、お気を付けください。
通常容器はしっかり密閉して、日の当たらない風通しの良いところで保管いただければと思います。
3週間前まで勘違いをしておりメタノールを水で薄めたものを霧吹きで全身に使っておりました…。
現時点で何も変化はないのですが、いつごろまで中毒については気を付けておかなければならないのでしょうか。
また、メタノールがなくなったため、洗いもせずエタノールを入れてつかっているのですが、その点は大丈夫なのでしょうか。
メタノールがもたらす影響は目に見えるものだけではありません。
「使っているけど大丈夫」という人もおられるかもしれませんが、使用状況はそれぞれ異なるということ、耐性にも個人差があることから、一度お医者様にかかられることをお勧め致します。
また、容器を洗われていないと、どうしてもメタノールが含まれるエタノールということになりますから、弊社としてはご使用をおすすめできません。ご了承ください。
質問No.31~40
※「▼」をクリックすると質問が表示されます。
今日、実験にてメタノール(濃度99.8%)を大量に零してしまい、その後片付けの際に瞬間的ではあるのですが、大量に高濃度のメタノール蒸気を吸引してしまったと思います。
現在、応急処置でお酒を大量に飲んでいるのですが、こういったケースで失明や視力低下など重症化することはありますか?
メタノールの毒性に関する体の反応には個人差がありますので、応急処置をなされたとはいえ、ご心配であればお医者様に診ていただいて診断していただいてください。
メタノールとエタノールで炎上しやすいのはどちらですか?
キャンプで着火剤としてメタノールのジェルが売っていますが、エタノールの手洗いジェルなどを着火剤として使用しても問題ないものでしょうか。
手洗いジェルなら応急キットに入っているのでわざわざ着火剤を用意しなくてもいいのかと思ってお伺いしました。
メタノールもエタノールも引火点はほぼ同じですので、メタノールの代わりとしてエタノールをお使いいただくことは可能ではありますが、除菌用のエタノールやジェルは濃度調整のため水分が含まれておりますのでこの水分があるせいで、着火剤よりも着火しづらくなっていますので、着火剤の代わりとしてお使いいただくことはおすすめできません。
皮膚表面への付着や気化したメタノールの吸引による身体への害はどれほどのものなのでしょうか。
少し匂いが分かるくらいに吸引してしまうのも危険なのでしょうか。
油汚れを落としやすいという理由で勉強机やスマホの画面をエタノールを付けたティッシュで拭いていたのですが、エタノールがなくなった際にアルコールランプ用として所持していたメタノールを代わりに使ったところ意外と使えたのでコロナ禍に入ってからも重宝して使用しています。
もともとメタノールの毒性は知っていたのですが、3分ほど手に付着した状態で清掃・気化した匂いを嗅いでいるうちに毒性が怖くなって質問させていただきました。
メタノールの毒性に対する耐性は個人差がございますので、一概には申し上げられませんが、視神経への影響や生殖毒性などがあります。
ご心配な場合お医者様にかかることをおすすめいたします。
おおまかにメタノール10%、水90%の廃液があります。この中のメタノール分を取り除くと排水処理施設へ流すことができます。
メタノール10%分を取り除く方法はありますが?
この廃液を蒸留してメタノール分だけを揮発回収させれば、廃液に水だけが残ります。
メタノールの廃棄方法を教えて下さい。
お恥ずかしい話ですが、メタノール(99%含有)と書かれた古いボトルが出てきました。
500㏄のボトルに300㏄程残っています。どう処分すればよいか分からず困っています。
メタノールの処分方法に関しては、自治体によってご案内が異なるので、お住まいの自治体にお問い合わせくださいませ。
メタノールを混ぜて消毒液として販売しているとしたら、それを調べる方法はあるでしょうか? 重さとか他の何かと混ぜるとか、燃やしてみるとか?
昨日こんなニュースを見まして、失明を引き起こす可能性がある危険な手指消毒液が製造されていると公的機関が警告
https://gigazine.net/news/20200624-toxic-methanol-found-hand-sanitizers/
消毒用アルコールとして販売されている物でも輸入品がほとんどで、アルコール70%と書かれていたけど30%だった、といったニュースは以前見ており、揮発性がいかにも少ないといった見分け方はあるようです。
怪しい商品を買わないのが一番なのですが、日本の有名メーカーの品は凄まじい高価かそもそも入手不可で辛い所です…。
メタノールが混ざっているかどうかを判定するにはガスクロマトグラフィーをかければわかるのですが、一般家庭で調べることは難しいかと思います。
販促になってしまい、誠に恐縮ではございますが、弊社のグループ会社「三協製薬」様のほうでエタノール系除菌剤の「エタノール除菌力」という製品を扱っておりまして、まだ在庫もございますので、よろしければ一度ご検討くださいませ。
メタノール95%の燃料用アルコールが残っているのですが、これを処分してもらうには「産業廃棄物」を取り扱っている業者に頼めば良いでしょうか?
自治体のサイトを見ても、「自治体では取り扱えません」と書いてあるだけなので、途方にくれています。
あと、ネットでは水を加えて少しづつ紙に吸わせ捨てれば良いともあったのですが…危険ですか?
管轄の自治体でお引取りいただけない場合は、業者に依頼されることをお勧め致します。
書いてくださった紙に吸わせる廃棄方法ですが、紙に吸わせることで、より燃えやすくなる危険性があります。また吸引のリスクも上がってしまい、メーカーとしてお勧めできません。
(例:ろうそくは蝋だけでは燃えないけれど芯があることで燃えます。メタノールは芯がなくても燃えますが、紙という芯があることでより燃えやすくなってしまいます)
今まで使っていたハンドジェルにメタノールが(49%)含まれていたことが判明しました。
重篤な健康被害は考えにくいというメーカーの見解が出ております。何かご助言いただければ助かります。お医者さんに行く場合も何かに行くのでしょうか。
今まで使っていたハンドジェルにメタノールが(49%)含まれていたためリコールするという連絡が来て、メタノールについて検索しておりましたところ、こちらのサイトに参りました。
30mlの物を二つ買って、小学4年生の娘が数週間使っておりました(ちょびちょびつかっていたため合計で10mlも使っていないと思います)。
香り付きの物だったため、「いい匂い~!」とわざわざしっかり吸引していました。今から思えば、私も少し使った際に、あまりに揮発性が高いことに違和感は持っていました。これは今後どう進めればよいのでしょう。
揮発性に関していえば、エタノールもメタノールもほとんど同じくらいの揮発性なので、使用しているうえで「これはメタノールかエタノールか」ということは判断ができません。
(弊社も、成分を確認する際はガスクロマトグラフィーをかけなければわかりません。)
お医者様にかかる場合は、まず普段かかりつけのお医者様にその旨をお伝えいただくことをお勧め致します。
実験室で器具の洗浄にメタノールを使っているのですが、これはどこの実験室でも行っていることなのでしょうか?
また、片目に違和感を感じているのですが、どういった病院に見てもらうべきなのでしょうか。
メタノールは安価なので、水溶性の汚れを落とすのに使用されることがあります。アセトンなども活躍しています。
正しく使用すれば便利な溶剤ですので、しっかり換気をし、火気に十分注意をなさって、保護具などをご使用のもとお使いください。
体に異変があるとお感じになった場合、まずはかかりつけのお医者様にご相談なさってください。
UVレジンの洗浄用として使用しても問題ないでしょうか?
UVレジンの洗浄用でいつもはエタノールを使用しているのですが、コロナの影響なのかどこにも売っていません。
その為、燃料用アルコールが代わりに使えると知り購入しました。しかし、メタノールが危険と言う事を知り不安の為お伺いです。
購入した燃料用アルコールの含有量はメタノール76.6%,エタノール21.4%,イソプロパノール0.3%です。
メタノールの誤飲は、使用者の誤飲ケースもありますが、使用していない方の誤飲ケースが非常に多くなっております。
そのためメーカーとしてはご使用いただきたくない製品です。
やむを得ずご使用になる際は有機溶剤用の防毒マスクやゴーグル、保護手袋を着用のもと、火気を避け、しっかり換気をなさったうえでご使用ください。
質問No.41~50
※「▼」をクリックすると質問が表示されます。
洗浄する際に使用した燃料用アルコールが染み込んだティッシュなどは燃えるゴミに出しても大丈夫なのでしょうか?
完全に乾いたら危険性はないとのコメントを見たのですが、この解釈で間違いはありませんか?
また、燃料用アルコールが完全に乾くまでは換気というか、玄関先など外に出しておいてもいいのでしょうか?それとも換気をしている部屋に完全に乾くまで置いておいた方がいいのでしょうか?
完全に乾いてしまえばティッシュにメタノールの残渣は残りませんので可燃ごみで大丈夫です。
お外に出しておいた方が換気的な面ではよいのですが、火気や高温には注意なさってください。
メタノールを洗浄後の水分置換に使用しています。エアブローも行いますので、局所排気は設置しております。
メタノールは劇物になりますので、量の管理は必要だと思いますが、洗浄中に水分が入ること、揮発することで残量が変わり、メタノールの濃度も薄まります。
こういった場合の台帳等の管理はどのように考えたらよいのでしょうか。
現行どのようなご使用、管理をなさっているのかわからないので、あくまで推測における一つの案ですが、缶やドラムからお出しになった段階で「使用」として計上されるのはいかがでしょうか。
社内でメタノールをハンドラップに入れて使用していますが、発生源の密閉対策につながりますか?
有機則の適用範囲に入るかどうかというのは、使用量の確認(1時間当たりどれくらい使用したか)と作業環境測定が必要になります。
使用量の減少・測定値の減少につながれば、対策として有効ということになります。
メタノール使用量でどれくらい揮発(飽和度、空気汚染 PPM)する大まかな計算式や方法があれば教えていただきたいのですが・・・
使用方法で揮発度合いは変わると思いますが、例えば室温25℃の無風状態で通常の200ccのコップにメタノールを満タンにそそぐと、時間当たりの揮発量、1立方メータ中の空気中の濃度はおおよそどのくらいになるのか知りたいです。
それが計算式でわかれば、弊社もとても助かるのですが、残念ながら、そのような計算式はございません。
実際に測定したり、使用量(使う前と使った後の実際の量)から計算する他ないのです。
物置から古いメタノール(99.3%)が出て来ました。
普段から燃焼用アルコールをアルコールストーブで使用していますが、こちらはメタノール(76.6%)です。99.3%のメタノールを、燃焼用に使用しても大丈夫なのでしょうか?
ご使用は頂けますが、炎の色が76.6%のものとは異なってきますので注意が必要です。
(76.6%のものは、安全のため火が点いているか否か分かりやすいよう、他成分などで調整されています)
メタノールについて検索していてこちらのサイトにたどり着きました。
自動車のウォッシャー液にメタノールが成分として含まれているのですが、ウォッシャー液のような液体が農地に流出してしまった場合、土壌や農作物にはどのような影響があると考えられるでしょうか?
他社様製品のSDSになりますが土壌中には排出されないように注意するような記述もございますが…。
メタノール自体は、微生物によって分解されますので、土壌への影響は大きくないと思いますが、土壌に排出してよいということとはまた異なります。
農作物への影響は分かりませんので、詳しくは専門家にお問い合わせください。
アルコールストーブでトルネードに燃焼するものはトルネードにならないものに比し、炎が高くなるのでアルデヒドが出やすいと書いてる方がいらっしゃいますが、燃焼方法でそのような違いはあるのでしょうか?
弊社では燃焼に関する細かな分析は行っておりません。
ユーザー様の方で燃焼に関する検証を行われている会社様もあるかとは思いますが、弊社には燃焼方式の違いによる気体発生の違いに関する知見がなくお答えすることができません。
木材の白カビについてご教示願いませんか。メタノールは白カビに有効ですか?
弊社ではメタノールが白カビに効力を持つかどうかという件に関する知見がなく、お答えすることができません。
先日祖父の家の片付けをしていたらお酒の容器にメタノールが入っていたらしく嗅いだときに物凄くアルコール臭かったのと傷口に触れてしまったのですがこの場合症状が出たりしますか?
調べたら生殖毒性もあると書いてあったのでその辺も教えてください。
例えば、飲酒した際、すぐに酔う人、全然酔わない人、アルコール消毒をした際、かぶれてしまう人、何ともない人がいるように、有機溶剤の耐性も個人差があります。
そのため、一概に申し上げることはできませんので、ご不安であればかかりつけのお医者様にご相談いただくのが最善でございます。
また、メタノールが入っていたのがお酒の容器であるということで、誤飲のリスクが非常に高くなっています。
メタノールが入っていると知らない人がお酒だと勘違いして飲んでしまう可能性がございますので、容器を変える、もしくは絶対に飲んではならないものであると一目瞭然の状態にしておいてください。
飲んでしまうと失明やその他健康被害のおそれがあり、大変危険です。
燃料用アルコール(メタノール95%エタノール5%)を買ったのですが少ししか使わず処理に困っています。
燃料として使う以外の方法があれば教えていただきたいです。安全な捨て方があればそれも教えていただきたいです。
燃料用なので燃料としてお使いください。(お力になれず申し訳ありません)
破棄の際は、自治体によって推奨方法が異なりますので、管轄の自治体様へご相談ください。
質問No.51~
※「▼」をクリックすると質問が表示されます。
メタノールが付着した布を誤って食器の上に置いてしまった場合、洗った食器を使用しても大丈夫でしょうか。
外国製のスマホ用カバーフィルムを購入したとき、貼付前の画面清掃用にアルコールクロスが付属していました。
クロスの小袋を開封すると、普段使用しているアルコール消毒液よりも強い臭気があり、海外製品ということもあり大丈夫かな?と思ったけれどあまり深く考えず使用したものの、すぐに何だか不安になり、クロスを触った手を洗ったのですが、恥ずかしながら流し台に洗う前の食器を置いたままで、洗った水が食器にかかってしまったかもしれません。
アルコールクロスにはほんの1分足らず触っていただけなのですが、指先の表面が軽くひび割れるくらい乾燥しており、もしかしたらこのクロスにはメチルアルコールが使われていたのでは?と思っています。
メーカーには薬剤の内容を問い合わせている最中なのですが、もしこれがメタノールだった場合、このような状況で、洗った食器を使用しても大丈夫でしょうか。
手を洗った段階では、まだ完全に薬液は揮発していなかったように思います。(指先にうっすら付着している状態)
水に混ざると揮発しなくなるとか、そのようなことはあるのでしょうか。(食器の表面に残ったりするのかなど……)
メタノールはエタノールよりもにおいが少ない物質のため、おそらくにおいが強いのであればIPA(イソプロピルアルコール)ではないかと思いますが、メタノールもIPAも水溶性のため、水で洗い流せば、流れ落ちてしまいます。
ご安心くださいませ。
もしご不安であれば、かかりつけのお医者様へご相談なさってください。
メタノールを50%含む溶液が霧状に噴射され、目に一瞬入ったような刺激がありました。すぐに目を水で10分程度洗いましたが、とても心配です。
目への刺激は特にありませんが、放置して大丈夫でしょうか?
溶剤への耐性は個人差がございますので、一概に申し上げることがございません。
かかりつけのお医者様にご相談ください。
メタノールを初期の段階から添加すればカビや雑菌が繁殖しずらい環境になりますか?その場合、どれぐらい添加すればよいでしょうか?
粉顔料を水で練って少量の酢酸ビニル樹脂と合わせて、空気中に触れる状態で保管しておくとドブの様な匂いがして腐ってしまいます。特に夏場、通気が悪い箇所なので。
塗料にはメタノールが含まれているのが多いので防腐の為なのかなと思いまして。
水の割合によっては、メタノールは雑菌の餌となってしまい、逆に菌が繁殖する原因となってしまいます。
塗料にメタノールが含まれることが多いのは、塗料を溶かす溶剤として使われているためです。
ラッカー塗料が腐らないのは、水が含まれていないためです。
防腐剤を入れるのがよろしいかと思いますが、防腐剤は弊社では取り扱いがないので、お手数ですが一度防腐剤メーカー様にお問い合わせくださいませ。
ガラスの撥水処理をするために研磨してからイソプロピルアルコールで洗浄すると聞いたのですが、イソプロピルアルコールの代わりにメタノールを使用する事は可能ですか⁉️
可能ですが、メタノールはIPAに比べて毒性が高いので、弊社ではお勧め致しません。
シンナーの多くにはメタノールが含まれていますが、眼への危険性など有害性があるにも関わらず、プロパノールやブタノールに置き換わったりしていないのは、代替できない優れた洗浄性能があるのでしょうか?
主にメタノールが含まれているシンナーはラッカーシンナーや洗浄用シンナー、それに近いアクリルシンナーなどが挙げられます。
これらにおいては、プロパノールやブタノールよりもメタノールは溶解力が高い、ということと、乾燥スピードが速いこと、メタノールが安価であるということが大きいのではないでしょうか。
物性がメタノール可溶性とある場合、エタノール可溶性でもあると理解して良いのでしょうか?
自分で育てたローズマリーがあります。カルノシン酸の新型コロナウイルス感染予防効果についての東京工業大学のプレスリリースを読みました。
in vitroな実験結果のようですし、作用機序からすると、実際にその恩恵に預かるにはちょいちょいこまめに気道にカルノシンが接しなくては意味が無いのではと考え、マスクスプレーを作ることにしました。家人は首都圏に毎日出向くし在宅勤務など不可能なサービス業だからです。
ローズマリーはよく油で熱滲出しますが、酸化と無縁にかつ楽に作りたくて、エタノールでチンキをと考えました。カルノシン酸は脂溶性か水溶性かわからなかったのですが、80%エタノールならどちらでも可と考えました。その後資料に当たっていくうちにメタノール可溶性であることは分かりました。
メタノールとエタノールの基本的な違いは分かるんですが、同じアルコールの仲間だからといってメタノール可溶をエタノール可溶と読み取って良いものかはいまだに分かりません。ですのでそこを教えて頂けますか?
序に派生的な質問も一つ、出来ればお願い致します。
食品の酸化防止剤としてよくローズマリー抽出物が使われてますが、メタノール可溶性だからといって毒性のあるメタノールで抽出した物を使って大丈夫なんだろうかと思ってしまいました。厚労省が禁止してればやらないと思いますが…。
抽出後に揮発してしまえば食品に加える物にもメタノールは使われるんでしょうか?
エタノールとメタノールは同じアルコール類で分子量も近いので、似た溶解性を示します。
また、食品に接する物質は、食品添加物でないといけませんので、メタノールで抽出したものは使用できません。
アルコールランプやハンドラップ等でのメタノールの使用について教えてください。
通常の使用状態で有れば、容器の中にメタノールがなみなみと入っています。この状態は劇物としての量の管理は必要でしょうか。
アルコールランプ同時に、アルコールランプの補充用の容器や洗浄瓶とうにいれて保管する場合も投入済みの扱いで良いと思われますか。
弊社では燃焼に関する細かな分析は行っておりません。
ユーザー様の方で燃焼に関する検証を行われている会社様もあるかとは思いますが、弊社には燃焼方式の違いによる気体発生の違いに関する知見がなくお答えすることができません。
ブルーレイレコーダーがディスク読み込まなくなったので、ピックアップを綿棒で洗浄しようと考えました。
レコーダーは11年目なのでメーカー修理が終了しています。自宅にメチルアルコール(99.5%以上)がありますが、使っても良いものでしょうか。
ディスクによっては、メチルアルコールが侵してしまう場合がございます。
侵さない場合もありますが、ディスクのメーカー様へお問い合わせ頂くのが確実です。
もしメタノールがディスクを侵さない場合、保護マスク(不織布のマスクではない)や保護メガネをかけたり、換気のよく、火気のない場所で作業ください。
釣竿塗装の前処理で脱脂にメタノール使用してますが、ゴム手に穴が開いてるの気付かずに使用すると手の皮が2~3日後には剥けてしまいます。
それほど油分を分解するわけで脱脂にはメタノールが良いと思い使用してますが、人体に悪影響なのが気がかりです。
人体にあまり影響がなく脱脂に向いてる溶剤があれば教えてください。
エタノールであれば、メタノールと同様アルコール類なので、同じような用途にご使用いただける上、安全性もメタノールよりも高くご使用いただけます。
ですが、通常エタノールは酒税が掛かってしまいますので、販促になってしまいますが、弊社エタコール7であれば、エタノールがベースでありながら、かつ酒税のかからないタイプなのでお手頃かと思います。
カテゴリーから探す
キーワードから探す