お知らせ・お役立ち便覧 NEWS
化学用語解説
2016.12.14
用語解説

キーボード左下の「Ctrl」と「F」のキーを同時押しして、調べたいワードを入力すると、そのワードの書いてあるところまでジャンプできます。
あ行
アクリル樹脂
アクリル酸、アクリル酸エステル、エタクリル酸、メタクリル酸エステルを主鎖とする共重合体のこと。熱可塑性アクリル樹脂や熱硬化性アクリル樹脂、変性アクリル樹脂などの種類がある。熱可塑性樹脂は単独またはセルロース系誘導体やロジン系樹脂などと併用しアクリルラッカーとして建築外装用、プラスチック用などに利用される。アクリル樹脂の主な用途は塗料の他に接着剤、印刷インキ、繊維加工用、皮革なめしなどである。また、アクリル樹脂は耐久性と透明性に優れ、水族館の水槽に使われている。
アニリン点
アニリン点(混合アニリン点)とは、油脂の溶解性をみる際用いられる指標の一つである。
試料(検査の対象として使う物質)と等しい容積のアニリンを混合して冷却した際、試料とアニリンが分離するときの温度のこと。一般的にアニリン点が低いほど、溶解性が高いと言える。但し、アニリンがある温度で分離する溶剤でないと測定はできない。
| 溶剤 | アニリン点 |
| ノルマルヘキサン | 59 |
| シクロヘキサン | 30.2 |
| ノルマルヘプタン | 69.3 |
| ベンゼン | -30 |
| トルエン | -30 |
| ホワイトガソリン | 55 |
| 灯油 | 78 |
アルコール
炭化水素の水素原子をヒドロキシ基(-OH)で置き換えた物質をアルコールと総称する。
<工業用アルコール例>
●メタノール(CH3OH)
●エタノール(C2H5OH)
●イソプロピルアルコール(CH3CH(OH)CH3)
●ノルマルプロピルアルコール(CH3(CH2)2OH)
また、アルコールと言って「エタノール」を指すこともある。
<アルコールの種類>
●合成アルコール
●発酵アルコール
<アルコール事業法>
アルコール事業法の対象になるアルコールはアルコール分が90度以上(温度15℃で測定)のもののことで、これは以下の二つに分類される。
①一般アルコール
加算額が含まれない安価なアルコール。使用には事前許可が必要。
使用などの記帳、使用数量等の定期報告が必要。
②特定アルコール
酒類への不正使用を防止するため加算額が設けられるアルコール。使用に関する記帳などの手続きは必要ない。
詳しくはこちら・・・「エタノールについて」
異性体
分子式は一致しているものの、分子構造が異なるもの同士を「異性体」と呼ぶ。
異性体には2タイプあり、ひとつは「構造異性体」と呼ばれ、分子式は一致しているが構造式が一致しない(順序や構造が異なる)ものがある。もうひとつは「立体異性体」と呼ばれ、互いに鏡に映したような関係にあるものをさす。
構造異性体は、化学的性質が大きく異なるものが多く、立体異性体は構造異性体ほどではないが、多少の違いは生じてくる。
引火点
引火点とは、火元を近づけた際に着火し燃焼する最低の温度のことを言う。液体は引火点に達せずとも蒸発をしているが、濃度が十分ではないため引火はしない。液体温度が上がることによって、蒸発量が増え、可燃性の蒸気の濃度が上がり、引火する。
なお、消防法は引火点に準じてレベルが定められている。
ウレタン樹脂
ウレタン樹脂とは、ウレタン結合を有する重合体の総称である。ポリウレタン、ウレタンゴムとも呼ばれる。イソシアネート基と水酸基を有する化合物の縮合により生成される。
ウレタン樹脂の硬化剤であるポリイソシアネートは、水との反応性が非常に高いため、高湿度や水分の混入に注意しなければならない。
<特徴>
●メリット●
低弾性から高弾性率まで幅広い物性が得られる
抗張力や耐摩耗性、耐油性に優れる
耐候性、耐水性、耐薬性に優れている
●デメリット●
耐熱性は他の合成ゴムに比べて低い
水分による加水分解や空気中の窒素化合物、塩分、紫外線、熱、微生物などの影響で徐々に分解される
<用途>
・ウレタン塗料(水溶性ウレタン塗料)
・ウレタン接着剤(ホットメルト接着剤)
・ウレタンフォーム(発泡ウレタン)
・ウレタンスポンジ
・シーリング材
・充填剤、断熱材
・防音材
・繊維製品(ストレッチ素材)
・革製品
・自動車部品(インシュレーター、クッション)
ウレタンは塗膜が固まると強固なため、剥離は容易ではない。
ウレタン塗装
ウレタン塗料は硬化剤を使う2液型仕様で、硬化剤を混ぜた時点ですぐに化学反応による効果が始まり、三次元の網目構造を形成して緻密な塗膜を作る。塗膜が柔らかく、密着性に優れ、価格、耐久性、機能性などにおいてほかの塗料よりバランスがよく、汎用性のある塗料として外壁塗料、床材塗装に多く用いられる。一方で、防汚性や紫外線に対する耐久性はシリコン系塗料と比べると劣る。耐久年数は8~10年で、アクリル塗料系(耐久年数5~8年)よりは長い。
ウレタンフォーム
ポリイソシアネート化合物と水とを反応させ、その際発生する炭酸ガスによって発泡させて作る発泡体のこと。
結露防止、凍結防止に使用されるほか、断熱材や特殊造形・発砲造形、車のエアロとして、また、FRP・DIYの補修や補強に使われている。
ウレタン防水
ウレタン防水とは、液状のウレタン樹脂を塗りつけ硬化させ、ゴム状で弾性のある防水層を形成させる工法のこと。手法には2液型が一般的であるが、他にも湿気硬化型の1液型、スプレー方式による超速硬化型などがある。
SP値
SP値とはSolubility Parameterの略で、溶解性パラメーターのことである。溶媒・溶質間に作用する力は分子間力のみと仮定し、分子間力を表す尺度として使用される値である。物質には固有のSP値があり、SP値の近い物どうしはよく相溶するという原則がある。油性加工油のSP値は7~8ほどである。
| 溶剤名 | SP値 | 溶剤名 | SP値 |
| ノルマルヘキサン | 7.3 | MIBK | 9.6 |
| シクロヘキサン | 8.2 | 塩化メチレン | 9.7 |
| 酢酸イソブチル | 8.3 | アセトン | 9.9 |
| 酢酸ブチル | 8.4 | イソブタノール | 11.2 |
| エチルベンゼン | 8.8 | ノルマルブタノール | 11.3 |
| キシレン | 8.8 | IPA | 11.4 |
| トルエン | 8.9 | ノルマルプロパノール | 12.4 |
| 酢酸エチル | 9.1 | エタノール | 12.7 |
| ベンゼン | 9.2 | エチレングリコール | 14.5 |
| トリクロロエチレン | 9.2 | メタノール | 14.5 |
| MEK | 9.3 | 水 | 23.4 |
エポキシ樹脂
エポキシ樹脂とは1分子中に2個以上のエポキシ基を有し、硬化剤によって三次元化する化合物の総称である。エポキシ樹脂はエポキシポリマー(主剤)と硬化剤が混ざり反応することで網目状の架橋構造を持つ硬化物が生成する。
エポキシ樹脂には1液型と2液型があり、1液性のものはあらかじめ主剤と硬化剤が混ざっており、加熱することで硬化剤が反応し硬化する、2液性のものは主剤(エポキシモノマー)と硬化剤を撹拌すると硬化するものである。
<特徴>
●メリット●
優れた接着性(金属・ガラス・木材・コンクリートなど)を持つ
耐食性・耐熱性・耐薬品性・耐水性・耐候性を持つ
硬化中に放出される揮発分がなく、硬化時の体積収縮が少ない
優れた強度と強靭性を持つ
電気絶縁性に優れており、電気を通さない
●デメリット●
靭性(破壊に対する感受性や抵抗値)が低い
紫外線に弱く、白く劣化する
低温下では硬化が遅い
<用途>
・エポキシ塗料…自動車用電着塗料、船舶・橋梁用重防食塗料、飲料用缶の内面塗装用塗料
・エポキシ接着剤…車両・航空機用接着剤
・家電製品、パソコン、携帯電話などの電子分野(積層板、半導体封止材、絶縁紛体塗料、コイル含浸用等)
・ゴルフクラブやテニスラケットなどのスポーツ用品用複合材料
エポキシ樹脂は硬化時に架橋構造を形成するため、硬化後の剥離・洗浄は容易ではない。
エマルション(エマルジョン)
水と油のように通常では混ざり合わない液体の一方を微細化し、もう一方の液体内に分散させた溶液のこと。分離している2つの液体をエマルションにすることを「乳化」といい、乳化する作用をもつ物質を「乳化剤」という。身近なエマルションとして、アクリル絵具、マヨネーズ、接着剤などがある。
か行
CAS№
CAS№(キャスナンバー)とは「CAS登録番号」の略称で、化学物質を特定するため、アメリカの学術専門団体が発行している世界共通の番号のことである。個々の化学物質に固有の識別番号を振ることで、物質名に別名があっても、CAS№を見れば同じ物質であるかが判断できる。日本でも、CASの代理店業務を行い、CAS登録番号取得を行っている団体がある。その物質の登録数は2017年の段階で1億3000万を超える。
凝固点
凝固点とは液体が固体になる温度の事を言う。多くの場合は融点と一致する。
極性
洗浄分野で用いられることの多いワードで、分子内に存在する電気的な偏りのことを表す。極性の有無によって加工油と洗浄剤の相性があり、極性のない加工油と、同じく極性のない炭化水素溶剤は相性よく溶け合うが、極性のある水溶性の加工油(水が極性を持つ)は極性のない炭化水素系とは相性が悪く、溶け込まない。
KB値
KB値とは、カウリブタノール値の事で、試料の油脂飽和力を示す指標である。数値が大きいほどその試料は多くの油脂を溶解できると言える。
測定方法は、まず、カウリ樹脂ブタノール溶液を一定量フラスコに入れ、活字用紙の上に置き、試料を滴下していきます。そして濁りが生じて活字が読めなくなった時の溶剤のml数を表す。但し、カウリ樹脂ブタノール溶液はアルコールやグリコールには際限なく溶けるため、あくまで指標であるという認識が必要である。※右図は炭化水素でのKB値である(パラフィンで25~40、シクロパラフィンで50前後、芳香族で100前後である)
※KB値の測定はカウリ樹脂をブタノールに溶解させるため、アルコール、ケトン、エステルなどの極性溶媒では非常に大きな値となり、溶解性を判断する指標にはならない。
| 溶剤名 | KB値 |
| ノルマルヘキサン | 30.5 |
| シクロヘキサン | 60 |
| ベンゼン | 107 |
| トルエン | 105 |
| キシレン | 103 |
| 塩化メチレン | 136 |
| トリクロロエチレン | 130 |
| テトラクロロエチレン | 90 |
| 1-ブロモプロパン | 125 |
合成アルコール
化学反応によって目的のアルコールを生成させたもの。一般的に合成アルコールは合成エタノールのことを指す。エチレンを原料とし、水蒸気と直接反応させて製造している。用途としては化学工業用に限定され、洗浄剤、化粧品、医薬品等に使用される。
さ行
生分解性
物質が微生物によって分解される性質であることをいう。微生物の働きにより、分子レベルまで分解し、最後は二酸化炭素と水となる。
相溶性
二種類以上の物質が相互に親和性を有し、完全に混ざり合う性質やその作用のことである。逆に全く混合せず分離してしまうことを「非相溶性」という。
二種類以上の物質に、親和性があるかどうかを判断する際は、極性やSP値を指標とする。
(たとえば加工油は極性のないものが多いため、同じく極性のない炭化水素系に溶けること、
また、加工油と炭化水素系のSP値が近いことからも、互いに相性の良いことが分かる)
た行
炭化水素
炭化水素とはその名の通り、炭素原子と水素原子のみでできた化合物のことを言う。これは分子量に応じて気体、液体、固体の状態を取る。
<分類>
◆パラフィン(=アルカン)
一般式がCnH2n+2で表される鎖式飽和炭化水素の総称。
パラフィンは分子の形状からノルマルパラフィンとイソパラフィンに分類される。パラフィンは無臭に近く、
また毒性も極めて低く安全性の高い物質である。洗浄性能(溶解力、脱脂力)についてはナフテン系(=シクロパラフィン)や芳香族系に比べると劣るが、金属加工後の加工油には十分な効果を発揮する。
●ノルマルパラフィン(ノルパラ)
分子が枝分かれしていない炭化水素のもの。
直鎖型飽和炭化水素ともいい、ノルマルヘキサン(n-ヘキサン)、
ノルマルヘプタン(n-ヘプタン)などがこの種類にあたる。
●イソパラフィン(イソパラ)
分子が枝分かれしている炭化水素のもの。分枝型炭化水素とも言い、2,3-ジメチルブタン、イソオクタンなどがこの種類にあたる。
◆シクロパラフィン(ナフテン)
分子が環状になっている炭化水素のもの(CnH2n)。環状飽和炭化水素ともいい、シクロヘキサン、シクロペンタンなどがこの種類にあたる。
◆芳香族(アロマティクス)
ベンゼン環のような構造を有する炭化水素のもの。環状不飽和有機化合物(二重結合を含んでいる)の一種で、
ベンゼン、トルエン、キシレンなどがこの種類にあたる。
<特徴>
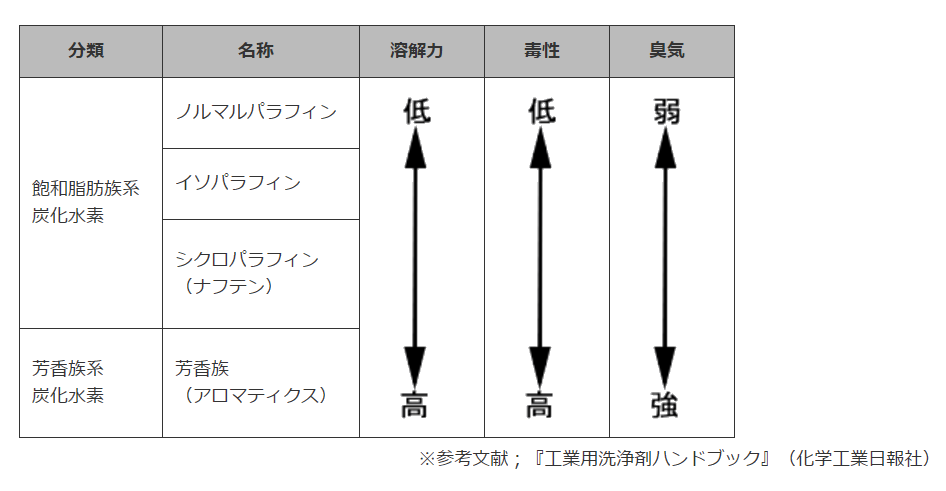
な行
燃焼点
燃焼点とは、ある物質が燃焼し続けるのに必要な最低液温のこと。基本的に、引火点よりもこの数字は高い。
は行
発火点
発火点とは着火温度とも言われ、自然発火温度のことである。この温度以上になると、火元がなくても自ら発火する。燃焼点よりも発火点の方が高くなるのが一般的である。
発酵アルコール
デンプンや糖蜜を原料とし、糖化、発酵後、蒸留して得られたものである。一般的に発酵エタノールのことを言う。用途としては酒類、その他食品、化粧品、洗剤、工業用途など、多岐にわたる。
比重
比重とは、ある物質の質量と基準となる水や空気の質量との比である。ある物質が液体や固体の際は水を基準とし、気体の際は空気を基準とする。単位は比であるため、ない。ある物質が、水の何倍の質量をもつか、という数値になる為、比重が1よりも大きい物質は水に沈み、1よりも小さい物質は水に浮く。密度とはまた異なる数値である。
沸点
沸点とは、別名沸騰点とも呼ばれ、その名の通り、沸騰し始める温度を示す。気圧によって沸騰する温度は変わってくるため、通常1気圧の際の沸騰し始める温度を表している。よく、固体が液体になる温度を示す「融点」と照らして、「液体が気体になる温度」だと誤解する人が多いが、沸点前でも液体は気体化する。bpと略されることがある。
フラックス
フラックス(flux)とは、松ヤニなど植物性の天然樹脂に薬剤を加えたもののこと。はんだ付けの際、あらかじめ基盤などの母材に塗っておくと、溶けたはんだの広がりがよくなるため綺麗に仕上がりやすく、はんだ不良の軽減が期待できる。
【フラックスの主なはたらき】
- 熱の伝導を妨げる酸化物が、基板などの電子部品の金属表面、あるいははんだの表面にできるため、それらを取り除きはんだづけを円滑にする
- はんだの表面張力を低下させることで粘り気を弱くし、はんだの流れ(濡れ)を良くする
- 固まったはんだ、母材、部品の表面をフラックスが覆うことで再酸化を抑制して不良を防ぐ
一般的な「糸はんだ」の中央にはフラックスが内臓されているが、それだけでは不十分の場合はフラックスを追加してはんだ付けをする。ただし、必要以上の塗布は、母材の金属の腐食や、水分を吸収し絶縁性が低下するなどの不具合発生の原因になる可能性があるため、最終的にはIPAなどのフラックス洗浄剤で洗い流す。
ま行
や行
融点
融点とは固体が液体になる温度のことを言う。多くは凝固点と数値が一致する。物質によって融点は異なり、他の物質が含まれていると融点に変動が見られるため不純物含有の有無を確認したり、物質の同定に利用することができる。
ら行
REACH
REACHは「Registration, Evaluation, Authorization and Restriction of Chemicals」の略で、2007年6月より実施されたEUにおける化学物質とその使用を管理する規則である。人の健康と環境の保護、EUの化学産業の競争力向上などを目的としている。年間の製造・輸入量が事業者あたり1トンを超えている化学物質が対象となる。
リスクアセスメント
「リスクを洗い出し、分析することで、リスクの評価を網羅するプロセス」のことをリスクアセスメントと言う。
ここでのリスクアセスメントとは、労働安全衛生マネジメントシステムに関する指針「危険性又は有害性等の調査及びその結果に基づき講ずる措置」の実施の事を言い、平成18年4月1日以降、努力義務化されている。また、その具体的な進め方に関しても、労働安全衛生法第28条第2項で明記されている。
RoHS指令
RoHS指令とは2006年7月よりEUで施行された指令で、電子機器における特定有害物質(鉛や水銀など)の利用を制限させるものである。制限することで、リサイクルの容易化、廃棄物の無害化・削減を目的としている。EU外からEU内への輸送も規制が生じており、注意が必要となる。
また、この指令の施行後、EU以外の国でもこれに準ずるような法令が見受けられる。
例)日本「電気・電子機器の特定化学物質の含有表示法」
レジストとは
レジストの名前の由来は『resist(処理に耐える)』から来ている。サンドブラストなどの物理的処理や、エッチングなどの化学的処理に対する保護膜や、保護膜の形成に使用される物質のことを指す。処理したい物の表面の一部をあらかじめ樹脂などで保護することで、保護していない部分だけ加工を施すことができる仕組み(保護膜は処理後、剥離する場合とそのまま残す場合がある)。用途や塗り分けの方法によって以下のような分類がある。
- フォトレジスト
- スクリーン印刷レジスト
- エッチングレジスト
- メッキレジスト
- ソルダーレジスト
わ行
カテゴリーから探す
キーワードから探す