お知らせ・お役立ち便覧 NEWS
有機溶剤
2018.02.27
テトラヒドロナフタレン(THNA)について
テトラヒドロナフタレンとは
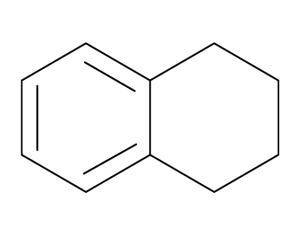
テトラヒドロナフタレンとは、略して「THNA」、別名「テトラリン」とも呼ばれる、芳香族炭化水素の液体です。
その名に「ナフタレン」という言葉が付くように、防虫剤としても有名な「ナフタレン」とよく似た構造をしています。
ナフタレンは2つのベンゼン環が1辺を共有した構造ですが、テトラヒドロナフタレンは、2つの環のうち、片方の環が水素化されて飽和している構造です。
製造する際にも、ナフタレンを白金触媒のもとで二重結合に水素を付加して作っています。
テトラヒドロナフタレンの特徴・用途
テトラヒドロナフタレンは、他の有機溶剤によく溶けるので、塗料やゴム、接着剤などの溶剤として使用されています。
また、油への溶解力も高く、機械部品などの油脂の洗浄にも最適です。
特にテトラヒドロナフタレンは浸透力が大きく、通常の溶剤では洗浄しにくい細かい部分の洗浄を得意としています。
他にも、医薬中間体を作る際の反応溶媒としても用いられています。
毒性について
最初に登場した、「ナフタレン」は特定化学物質障害予防規則(通称:特化則)に指定されていますが、テトラヒドロナフタレンは毒性が低く、特化則には該当していません。
そのため、有害性の高いトルエンやキシレン、ジクロロメタン、酢酸エチル、MEK、アセトンなど、他の有機溶剤の代わりの溶剤(代替溶剤)として、活躍が期待されています。
関連記事
カテゴリーから探す
キーワードから探す